Organic entities require numerous natural and inorganic substances to finish their life cycle. All such substances which are taken from outside comprise their nutrition. Based on their dietary necessities, life forms can be characterized as heterotrophs and autotrophs.
All non-green plants and creatures, including individuals, are heterotrophs.
Autotrophic green plants acquire their nourishment from inorganic substances which are available in the soil as minerals, which are known as mineral components or mineral supplements, and this kind of nutrition is called mineral nutrition.
Nitrogen Cycle
Nitrogen is the most basic component. Aside from carbon, hydrogen, and oxygen, nitrogen is the most common component in living life forms. Nitrogen is found in fundamental mixtures like proteins, nucleic acids, development controllers, and numerous nutrients. Plants rival microorganisms for the restricted nitrogen that is accessible in soil. In this way, nitrogen is a restricting supplement for both normal and farming environments.
Nitrogen exists as two nitrogen molecules joined by an exceptionally impressive triple covalent bond (N ≡ N). N2 gas of the air is changed over into alkali by the course of nitrogen obsession. In nature, lightning and bright radiation give sufficient energy to change nitrogen over completely to nitrogen oxides (NO, NO2, N2O).
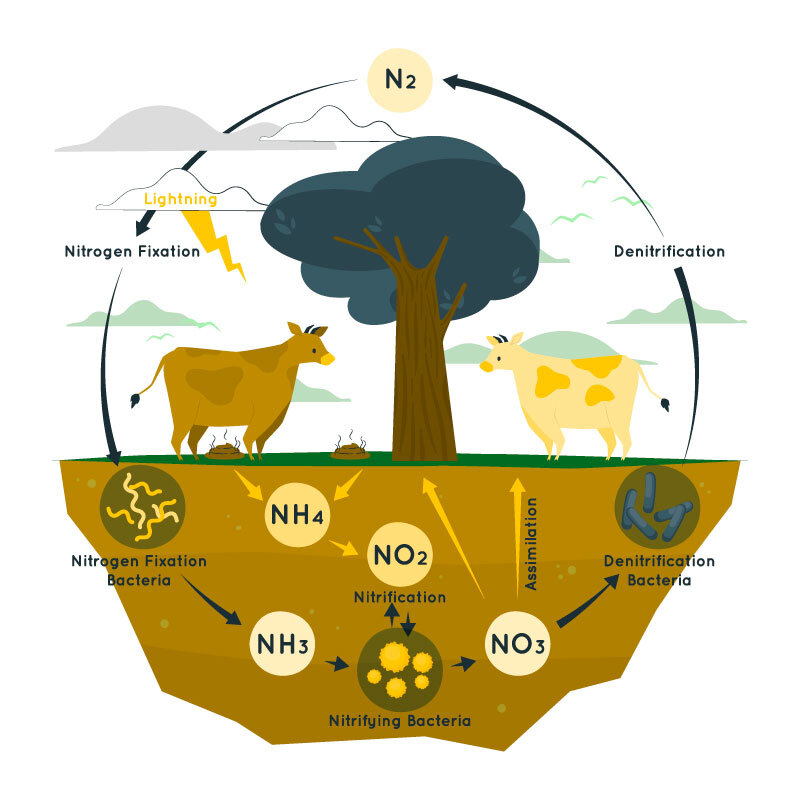
Modern ignitions, timberland fires, car debilitates, and power-creating stations are additionally the wellsprings of climatic nitrogen oxides. A standard stockpile of nitrogen for the plants is kept up through the nitrogen cycle. The nitrogen cycle is a customary course of nitrogen among living creatures. The nitrogen cycle comprises four cycles called nitrogen obsession, ammonification, nitrification, and denitrification.
Nitrogen Fixation
The principal phase of the nitrogen cycle is the change of the inert form of nitrogen gas (N2) into alkali (NH3) This response is catalyzed by the chemical nitrogenase, which is delivered by nitrogen-fixing microorganisms in the dirt Azotobacter is tracked down living openly in the dirt, while Rhizobium shapes a mutualistic relationship with the foundations of vegetables. Rhizobium structures knobs inside the plant roots and supplies smelling salts to the plant in return for carbs. Alkali (NH3) becomes ammonium (NH4+) when blended in with water, and this can be utilized by plants in the nitrogen cycle:
- A plant takes nitrogen from the dirt by retaining it through its underlying foundations. Nitrogen comes as nitrogen particles. At the point when the nitrogen is consumed by the plant, it is decreased to nitrite particles. Then, it becomes ammonium particles which can be integrated into amino or nucleic acids and into chlorophyll.
- At the point when a plant kicks the bucket or a creature bites the dust or when a plant or a creature ousts squander, natural nitrogen is then delivered. Microorganisms can change over this natural nitrogen into ammonium. They do this through an interaction called mineralization.
- Nitrogen gets into the seas because of spillover from groundwater or when it downpours. Nitrogen can likewise get into the sea through precipitation (downpour). Nitrogen in the water goes through obsession, which is by and large worked with by a kind of microscopic organism called cyanobacteria. After obsession, the nitrogen is in a naturally accessible structure that phytoplankton in the sea can utilize.
Ammonification
Alkali can likewise be delivered from natural wellsprings of nitrogen (for example amino acids) when separated by decomposers. As a plant or creature rots, saprotrophs will decay natural materials to deliver smelling salts (and ammonium particles). This interaction is known as ammonification and deliveries ammonium particles into the dirt which can be consumed by plants
Nitrification
Nitrification is the change of ammonium particles into nitrites and nitrates by nitrifying microorganisms in the dirt.
Nitrosomonas change over ammonium particles into nitrites, while Nitrobacter can change over nitrites into nitrates. These responses require oxygen and subsequently, the soil should be very much circulated air through to guarantee a rich stockpile of nitrites and nitrates. Nitrites and nitrates are more straightforward for plants to absorb and thus capable of a transcendent wellspring of nitrogen for plants
2NH2 + 3O2 ⇢ 2NO2¯ + 2H+ + 2H2O
2NO2¯ + O2 ⇢ 3NO3 ¯
Denitrification
Denitrification is a compound decrease process that converts nitrates (NO3-) into nitrogen gas (N2). It is completed by denitrifying microorganisms without any oxygen (for example anoxic circumstances). Nitrates can be utilized rather than oxygen as an electron acceptor during cell breath, creating nitrogen gas. This will just happen in oxygen-unfortunate circumstances like waterlogged soils and lessens the accessibility of nitrates to plants
Importance of Nitrogen Cycle
- Nitrogen gas (N2) is plentiful in our environment. Nitrogen is essential to structures that structure biochemicals which are key to all living cells.
- Nitrogen-fixing is the most common way of changing over nitrogen gas in the climate into smelling salts (NH3) and other nitrogen intensifies that can be integrated by cells into biochemicals.
- Not many cells can achieve this, and they are prokaryotes called diazotrophs
- Whole environments have come to depend on diazotrophs to fix nitrogen gas into usable structures like smelling salts (NH3) and nitrates (NOx).
- Plants can retain alkali (NH3) to make the nitrogen-containing biochemicals that creatures should eat.
- A few plants quite harbour nitrogen-fixing microorganisms in their roots, profiting from the smelling salts created straightforwardly in their tissues.
- Nitrogen-containing biochemicals made by living cells return to the climate as alkalis through metabolic waste and deterioration, and these smelling salts are reabsorbed by plants in the nitrogen cycle.
- In farming, we can add these mixtures as manure to the dirt to increment plant efficiency.
- Notwithstanding, compost and animal waste from ranches can overflow with the downpour causing unnatural algal development in encompassing streams, at last draining the oxygen (O2) required for a flourishing oceanic environment.
- Lightning is an actual interaction that produces smelling salts from nitrogen gas, and, a few microscopic organisms really separate nitrogen compounds, returning nitrogen gas to the climate and going full circle
Nitrogen Cycle in Marine Ecosystem
The essential supplements for marine essential creation are similar to ones you would use to treat your nursery: N-P-K or Nitrogen, Phosphorus, and Potassium. In marine frameworks Nitrogen is practically consistently accessible in similar overflow; all things considered, each time something kicks the bucket it places some amount of Nitrogen into the framework in addition each time a creature wipes out squander it places some amount of Nitrogen into the framework (for the most part as Urea or Ammonia). The only, difference between both cycles is the bacteria that do the nitrogen fixation.